electron configuration aluminum|Iba pa : Baguio A step-by-step description of how to write the electron configuration for Aluminum (Al). In order to write the Al electron configuration we first need to know the number of electrons.
The best mobile games use the medium to deliver engaging gameplay and gripping story, and 2021 saw a number of pocket-sized gems. From lovingly crafted RPGs to fresh spins on existing franchises .
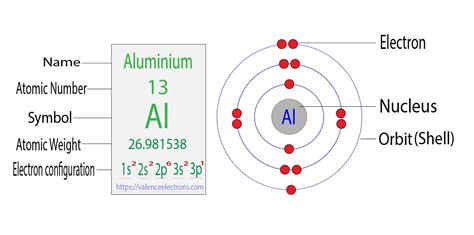
electron configuration aluminum,In order to write the Aluminium electron configuration we first need to know the number of electrons for the Al atom (there are 13 electrons). When we write the configuration we'll put all 13 electrons in orbitals around the nucleus of the Aluminium atom.The Electron configuration of aluminum is 1s22s22p63s23p1. Aluminum is one of the elements that make up the periodic table, which is distinguished by its symbol Al, and its atomic number 13.Learn how to write the electron configuration of aluminum through orbitals and orbitals, following different principles such as Bohr, Aufbau, Hund, and Pauli. S.
Mar 23, 2023 Electron Configuration of Aluminum. To find the electron configuration of an atom, you first need to know the number of electrons that it has. Since aluminum's .
A step-by-step description of how to write the electron configuration for Aluminum (Al). In order to write the Al electron configuration we first need to know the number of electrons.

The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Commonly, the electron configuration is used to .Iba paThe electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Commonly, the electron configuration is used to . Aluminium has atomic number 13 so the full electron configuration will be: 13Al 1s22s22p63s23p1. A shorthand way of writing this is to use the preceding noble .
Aluminium. 13. 26.982. Glossary. GroupA vertical column in the periodic table. Members of a group typically have similar properties and electron configurations in their outer shell. .Learn how to derive the electron configuration of aluminum (Z = 13) using the Aufbau principle and the Pauli exclusion principle. See the orbital diagram and the ground-state . 1 Answer. Aluminium has atomic number 13 so the full electron configuration will be: 13Al 1s22s22p63s23p1. A shorthand way of writing this is to use the preceding noble gas configuration by putting its symbol in square brackets in front of the valence electrons. In this case that is neon which is: The aluminium atom contains 13 electrons. The electronic configuration of the aluminium is expressed in the form of the diagram as given below-. 1s orbital having minimum energy is filled first, with a maximum capacity of two electrons. After 1s orbital, the 2s orbital is filled with a maximum capacity of two electrons.Q. Aluminum (Al) atom has ground-state electron configuration as : Q. What is electronic configuration .How to find the electronic configuration of metals as a ex.oxygen. Q. What are the valencies of the element given below. carbon. electronic configuration. 2,4. magnesium. electronic configuration. 2,8,2. oxygen. electronic .When their electron configurations are added to the table (Figure 6.29), we also see a periodic recurrence of similar electron configurations in the outer shells of these elements. Because they are in the outer shells of an atom, valence electrons play the most important role in chemical reactions. . Aluminum dication loses two electrons Al 2 . The atomic number of aluminum is 13. A neutral atom of aluminum has 13 protons and 13 electrons. The ground state electron configuration for aluminum is 1s22s22p63s23p1. A shorthand way to write the electron configuration, called noble gas notation, is [Ne]323p1. An atom in its lowest energy state is said to be in its ground state.
The first two subshells of the third shell are filled in order—for example, the electron configuration of aluminum, with 13 electrons, is 1s 2 2s 2 2p 6 3s 2 3p 1. However, a curious thing happens after the 3p subshell is filled: the 4s subshell begins to fill before the 3d subshell does. In fact, the exact ordering of subshells becomes more . The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +1 2 m s = + 1 2 ).
Let's find the ground state electron configuration of Aluminum! A single Aluminum atom has 13 protons and 13 electrons, but how do we know where Aluminum put.

Inner transition elements are metallic elements in which the last electron added occupies an f orbital. They are shown in green in Figure 5.1.6 5.1. 6. The valence shells of the inner transition elements consist of the ( n – 2) f, the ( n – 1) d, and the ns subshells. There are two inner transition series:
The electron configuration shows the distribution of electrons into subshells. This list of electron configurations of elements contains all the elements in increasing order of atomic number.. To save room, the configurations are in noble gas shorthand.This means part of the electron configuration has been replaced with the .The electron configuration for aluminum is 1s2, 2s2, 2p6, 3s2, 3p1. This is assuming the aluminum atom is a neutral atom in a grounded state.electron configuration aluminumElectron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point . Aluminium is used in a huge variety of products including cans, foils, kitchen utensils, window frames, beer kegs and aeroplane parts. This is because of its particular properties. It has low density, is non-toxic, has a high thermal .
electron configuration aluminum Iba paElectron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point . Aluminium is used in a huge variety of products including cans, foils, kitchen utensils, window frames, beer kegs and aeroplane parts. This is because of its particular properties. It has low density, is non-toxic, has a high thermal .They are helium, neon, argon, krypton, xenon, and radon. A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the remaining .
The Electron: Crash Course Chemistry #5. Video 2.6.2 2.6. 2: An overview of the role of orbitals in electron configurations and how to write electron configurations. The relative energy of the subshells determine the order in which atomic orbitals are filled (1 s, 2 s, 2 p, 3 s, 3 p, 4 s, 3 d, 4 p, and so on).
The noble gas configuration is a shorthand electron configuration for atoms. In chemistry, the noble gas configuration is a shorthand method of writing an atom’s electron configuration.The reason for using the noble gas configuration is because the full electron configuration becomes very long for atoms with high atomic . Introduction to electron configurations. Electron configurations describe where electrons are located around the nucleus of an atom. For example, the electron configuration of .
Aluminium is a chemical element with atomic number 13 which means there are 13 protons and 13 electrons in the atomic structure. The chemical symbol for Aluminium is Al. Electron Configuration and Oxidation States of Aluminium. Electron configuration of Aluminium is [Ne] 3s2 3p1. Possible oxidation states are -2; -1; +1; +2; .
Electron Configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table. The electron configuration for the first 10 elements. H .
electron configuration aluminum|Iba pa
PH0 · what is sodium complete electron configuration
PH1 · how to write electron configuration
PH2 · give the full electronic configuration for phosphorus
PH3 · give the full electron configuration for sulfur
PH4 · electron configuration chart
PH5 · electron configuration calculator
PH6 · complete the electron configuration for s
PH7 · Iba pa
PH8 · 1s 2s 2p orbital diagram